Pervasive Patient Monitoring - Take Two at Bedtime…
by Dirk Husemann and Michael Nidd
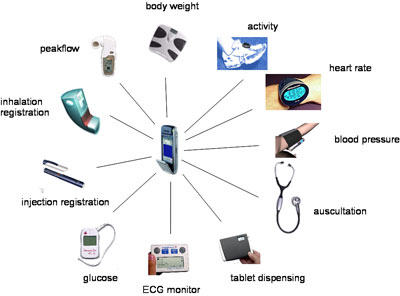
A team at IBM Zurich Research Laboratory Zurich has created the IBM mobile health toolkit for gathering measurement data from a range of devices, and present it to management software via a well-defined and easily implemented interface.
About 55% of all long-term patients in the US and in Europe, it is estimated, do not take their medication (either not taking the prescribed medication at all or more than 14 hours late) Around 12% of all hospital admissions in the UK are due to this non-compliance, the damage to the US taxpayer is an estimated USD 100 billion a year. Most of the patients that do not comply are simply forgetful (about 10% deliberately do not want to take the medication). For a lot of diseases it is important that doctor and patient work together: as the visit to the doctor provides just a snapshot in time of the patients health status, the GP has to rely on observations and self-measurements by the patient herself to provide a good diagnosis and select the right therapy. To capture this information patients are often asked to keep a patient diary. Unfortunately (another piece of bad news here), over 40% of all patient diaries are false. The British Medical Journal reports of cases where patients faked their patient diaries half an hour before their doctor's appointment while sitting in the doctor's parking lot, using ten different pens to make it look credible!
Gathering current patient medical data promptly and accurately is vital to proper health care. The usefulness of electronic data capture (EDC) has been demonstrated in applications such as the home monitoring of at-risk heart patients via devices that transmit blood pressure from the home to a central database. Removing transcription effort (and associated inaccuracies) alone is worth the institution of EDC; but the side benefit of timeliness offers the hope of identifying and responding to trends as they occur, perhaps preventing a dangerous event, instead of simply allowing its diagnosis after the danger has manifest.
|
The market is now at the beginning of the technology curve for EDC. A typical EDC offering is a full end-to-end solution, where a customer purchases measurement devices, data gathering and network technology, database, and visualisation/analysis software as a single package. For quality and cost to improve, work in the area must be allowed to specialise. To this end, our team has created an efficient and flexible toolkit, the IBM mobile health toolkit, for gathering measurement data from a range of devices, and present it to management software via a well defined, and easily implemented interface.
The Treatment
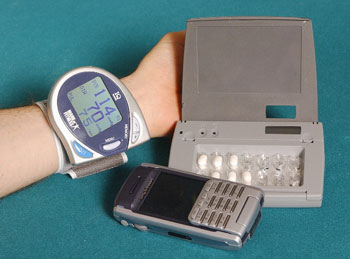
The IBM mobile health toolkit provides a Java-based middleware -- using J2ME MIDP 2.0 (Java Mobile Information Device Profile) and JSR 082 (Java APIs for Bluetooth) -- running on a personal (mobile) hub device to which sensors can connect wirelessly. We can perform local processing on the data, and forward the result to one or more fixed network connections. Data-handling modules can easily be added to the MIDlet suite (application suite compliant with Java Mobile Information Device Profile) on the hub, as can drivers for new sensor devices. While the data formats from these devices are usually proprietary, a popular physical medium for their transmission is Bluetooth. JSR 082, which standardised Bluetooth access from Java, means drivers for new devices are also small and easily written. A key feature of our mobile health toolkit is the use of an open, hierarchical event model segmented into \emph{domains.} Each piece of sensor information is normalized into a standardized event by the corresponding sensor driver before the information is forwarded to the toolkit; for example, a blood pressure cuff measures systolic and diastolic blood pressure values along with the current pulse --- the driver converts these sensor readings into two events: a standard blood pressure event and a standard pulse event. The use of a normalizing event model allows us to write downstream applications and adapters independent of the specific sensors used --- we can easily switch to other sensors models without having to modify existing applications.
|
Using a wireless link from the hub to the devices, as shown in the Figure, allows the hub to be placed in an unobtrusive location, saves the user from fiddling with cables, and saves the sensor manufacturer the trouble of finding an acceptable case location for the data connector. By requiring only Bluetooth, MIDP support, and a network connection from the hub, the range of suitable hardware choices for the hub extends from full PCs, through OSGi home gateway units, all the way to cellular phones.
The Status
By allowing different business partners to utilize one another's applications and sensors in new ways, the IBM mobile health toolkit has already enabled applications and solutions that were not previously possible.
We are currently in the third iteration of the IBM mobile health toolkit and have reached a state where the software is running rather reliably and is mature enough to be deployed in first applications. The current version is implemented completely in Java and already fully supported on such restricted platforms as J2ME MIDP 2.0 (requiring the JSR 082 Bluetooth Java API for sensor attachment).
Please contact:
Dirk Husemann and Michael Nidd,
IBM Zurich Research Laboratory, Switzerland/SARIT
E-mail: {hud,mni}![]() zurich.ibm.com}
zurich.ibm.com}

 This issue in
This issue in