Agent-Based Modelling applied to HIV/AIDS
by Ashley Callaghan
Through the use of agent-based computer simulations we hope to model both the spread of HIV/AIDS throughout the population and aspects of the immune response to HIV infection.
The devastating effects of the AIDS epidemic are compounded by its complex patterns of transmission. The rate of transmission and the demographic spread of the disease are influenced not only by direct factors such as age, marriage rates, number of sexual partners, sexual preferences, frequency of extramarital liaisons, etc., but indirectly by factors such as migration patterns, economic conditions, government policies and ultimately by the complex interactions between all these factors. It is these interactions and their evolution over time that provides a major difficulty in trying to predict the spread and social impact of the disease.
The rapid spread of HIV/AIDS throughout the world particularly in areas of sub-Sahara Africa has resulted in a desperate global need for an AIDS vaccine, but to date there has been little success in finding candidate vaccines that stimulate effective neutralising antibodies. In order to develop a viable vaccine the dynamics of the immune response to HIV infection need to be fully understood. In recent years much progress has been achieved in understanding HIV. However the exact mechanisms by which HIV causes AIDS remains unclear. When compared to other viral diseases, HIV infection and the progression to AIDS displays an unusual time evolution (i) initial contact with the virus results in a normal primary immune response, however instead of the virus being completely defeated it remains present in a low concentration; (ii) this phase is followed by the chronic phase. A long period of latency (2-10 years) during which time the CD4 T cell level slowly decreases; (iii) finally when the CD4 T cell count drops to approximately 20%-30% of the normal value, the immune system becomes unable to defend itself its host from opportunistic diseases and the patient generally dies within 2-3 years. If the mechanism that accounts for the slow decrease in CD4 T cells during the latent phase was properly understood it could be of enormous benefit towards designing an effective vaccine.
|
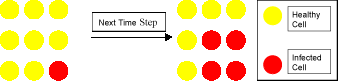
Agent-based simulations attempt to model the system under consideration by means of interactions between agents at a local level. An agent in an epidemic model would be an individual person, whilst in a model of the immune response an agent would represent an individual cell, whether that cell be a T cell, B Cell, virally infected cell, or any of the other cells that participate in the immune response. The agents then interact with each other based on simple rules. A simple rule in an epidemic model could be of the following form: a single woman will seek a single man within the same age group and then stay together and have children together with a certain probability. This may seem trivial but it allows us to model family structure, something that isn't realistically possible using mathematical techniques. In modelling the immune response to a virus a simple rule could be of the form: if a healthy cell has at least one infected neighbour it too becomes infected (see Figure). Out of such simple local interactions complex global phenomena can emerge.
Until relatively recently large scale simulations such as these weren't computationally feasible, but the advent of faster cheaper machines has lead to an increased level in the use of techniques such as parallel computing with the computational load spread amongst different processors. The processors may all reside on one machine such as a supercomputer or be coupled together in the form of a cluster.
This research is at a very early stage, but we hope to gain insight both into the population dynamics of the epidemic and the immune response to HIV infection. Hopefully this will enable us to determine useful control strategies against the spread of the epidemic, as well as giving us a greater understanding of the immune response. To date we have a simple model that allows us to model such things as family structure, extramarital liaisons, sexual preferences, migration, average lifespan, and different population growth rates. This model is implemented on a dedicated cluster consisting of 17 machines each with four processors. The area being modelled is divided into a number of regions, each of which represents a different geographical area. Each area is then simulated on its own processor with a control program operating on one of the processors to co-ordinate the work and to transfer data between the different processors. Distributing the work among a number of processors enables us to model large populations that wouldn't be possible on a standard desktop machine due to memory and speed constraints. This work will be continuing for the next three years and we hope to have interesting results within the next year
Please contact:
Ashley Callaghan, Dublin City University/IUC, Ireland
E-mail: Ashley.Callaghan![]() computing.dcu.ie
computing.dcu.ie

 This issue in
This issue in